Company profile
KreLo GmbH Medical Diagnostic was founded in 2004 at the Biotechnology Center in Ulm (TFU) and began as a Start up by spin off from University of Ulm.
The work therein was focused on research and development of IVD-Assay in autoimmunity.
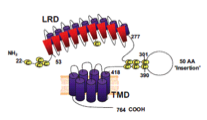
KreLo GmbH Medical Diagnostics developed the first test for the direct detection of Graves‘ disease inducing autoantibodies with a new technology, called bridge assay, on microtiter plates (sTRAb). This method was transferred to Immulit TSI of Siemens Healthcare. Further new technologies are based on paramagnetic beads technology.
History of research
History of pioneering in „In vitro“ diagnostic (IVD) development
1971 – 1993: At university of Ulm
1971: Replacing bioassays by radioimmunoassays (T4, T3, rT3, TSH)
1973: First description of low T3 (euthyroid sick) syndrom
1982: Proteomics (T3 responsive liver proteins)
1993: Detection of TSHR and T3 receptor mutation, functional as peripheral hyperthyroidism
1998 – 2004: At university of Ulm and personal enterprise INNOVATIVE MEDICINE
TSHR expression, antibody detection and quantification by immunoprecipitation (patenting)
2005 – today: At KreLo GmbH Medical Diagnostics
2005: TSHR antibody detection and quantification by bridge technology (patenting)
Since 2015: Paramagnetic beads application in bridge TRAb assay (patent application)
1998-2018: Cooperation with IVD companies (transfer of our prototypes)
Since 2017 Cooperation with Thermo Fisher Coating and handling of Paramagnetic Beads
Cooperation partners
Scientific collaboration with
- colleagues at Ulm and European universities (Birmingham, Kopenhagen, Bratislava, Bucharest, Tokio)
Innovative Medicine
- Scientific cooperation for transfer of intellectual property for creation for prototype of test system
Commercial cooperation with
- IVD companies for technology transfer
- Transfer of prototype to product development
- Siemens Healthcare (recently renamed Siemens Healthineers)
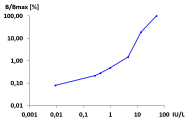
The sTRAb assay, created by KreLo GmbH was successfully transfered to Immulite TSI
Precondition given by the intellectual property and expertise as shown in the publication:
Frank C. U., Braeth S., Dietrich J. W., Wanjura D., Loos U. Bridge Technology with TSH Receptor Chimera for Sensitive Direct Detection of TSH
Receptor Antibodies Causing Graves’ Disease: Analytical and Clinical Evaluation. Horm Metab Res 2015; 47: 880–888“. Download https://www.thieme-connect.com/DOI/DOI?10.1055/s-0035-1554662Tozzoli R, D’Aurizio F, Villalta D, Giovanella L. Evaluation of the first fully automated immunoassay method for the measurement of stimulating TSH receptor autoantibodies in Graves‘ disease. Clin Chem Lab Med 2017; 55(1): 58-64.
Kiaei D, Conarpe C, Birmingham N, Lei J, Bitcon V. Clinical Evaluation of the IMMULITE 2000 TSI Assay: Siemens Healthcare Diagnostics, Tarrytown, NY, USA Download
https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context- root/wcm/idc/groups/public/@global/@clinicalspec/documents/download/mda3/mdgz/~edisp/immulite_tsi_clinical_eval_wp_final-04093195.pdf
Literature
Poster presented on international congresses
Franz et al., 2007: Direct quantification of stimulating TSH receptor autoantibodies in Graves‘ disease by the first in vitro assay suitable for routine clinical diagnosis (Deutsche Gesellschaft für Endokrinologie, Salzburg) Download (2007 DGE_Salzburg.pdf)
Franz et al., 2008: Direct epitope recognition assay for TSH receptor autoantibodies causing Graves’ disease demonstrates higher diagnostic accuracy than indirect assays based on TSH displacement. (American Assocciation of Clinical chemistry, Washington) Download (2008 AACC_Washington.pdf)
Bräth et al., 2015: Assay of TSH receptor stimulating immunoglobulins using paramagnetic microbeads as solid phase (International federation clinical chemistry, Paris) Download (2015 IFCC_Paris.pdf)
Bräth et al., 2015: Novel in vitro chimeric sTRAb assay measures thyroid stimulating autoantibodies (TSI) in serum of Graves’ disease patients (American thyroid assocciation, Orlando) Download (2015 ATA_Orlando.pdf)
Loos et al., 2017: Sera from patients with Hashimoto’s disease impair TSH signaling in a TSH receptor bioassay, and coexisting TRAb are identified as stimulating (Deutsche Gesellschaft für Endokrinologie, Würzburg) Download (2017 DGE_Wuerzburg.pdf)
Orginal Publication Of The Innovative Novel Bridge Technology For Double Direct Detection Of Autoantibodies In Graves’ Disease (sTRAb- DERA)
Frank C. U., Braeth S., Dietrich J. W., Wanjura D., Loos U. Bridge Technology with TSH Receptor Chimera for Sensitive Direct Detection of TSH Receptor Antibodies Causing Graves’ Disease: Analytical and Clinical Evaluation. Horm Metab Res 2015; 47: 880–888“. Download https://www.thieme-connect.com/DOI/DOI?10.1055/s-0035-1554662